
Hermosa chica con vestido dibujo a lapiz/ Como dibujar una chica/ Dibujos faciles paso a paso - YouTube
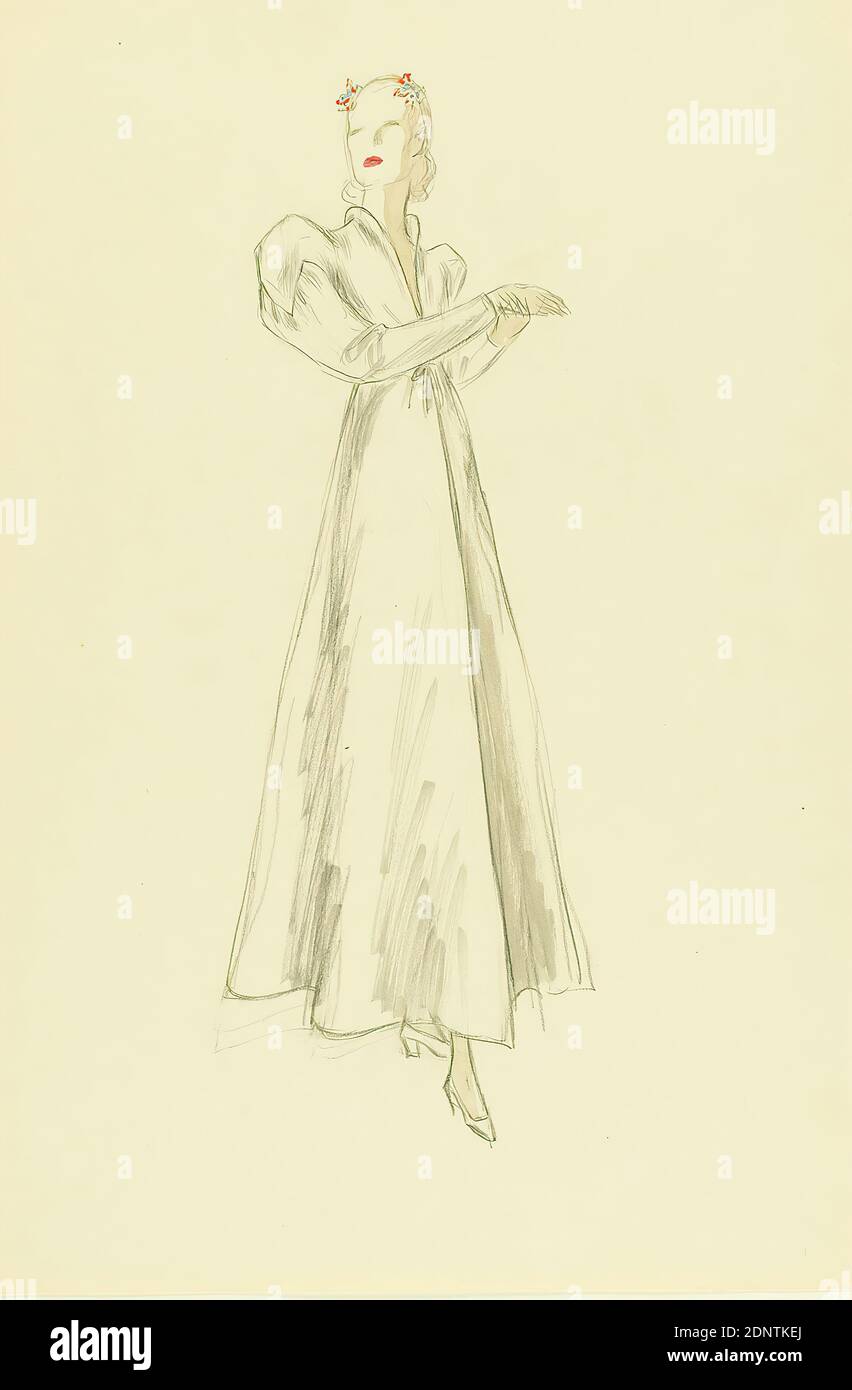
Ernst Dryden, dama con vestido blanco, papel, acuarela sin revestimiento, lápiz, acuarela, dibujo, acuarela sobre lápiz, total: Altura: 45.9 cm; ancho: 30.9 cm, dibujo, gráficos, ilustraciones, moda, ropa, moda femenina, vestido Fotografía

13 ideas de Vestidos a lapiz | dibujos de moda, dibujo de diseño de vestido, dibujos de diseño de moda
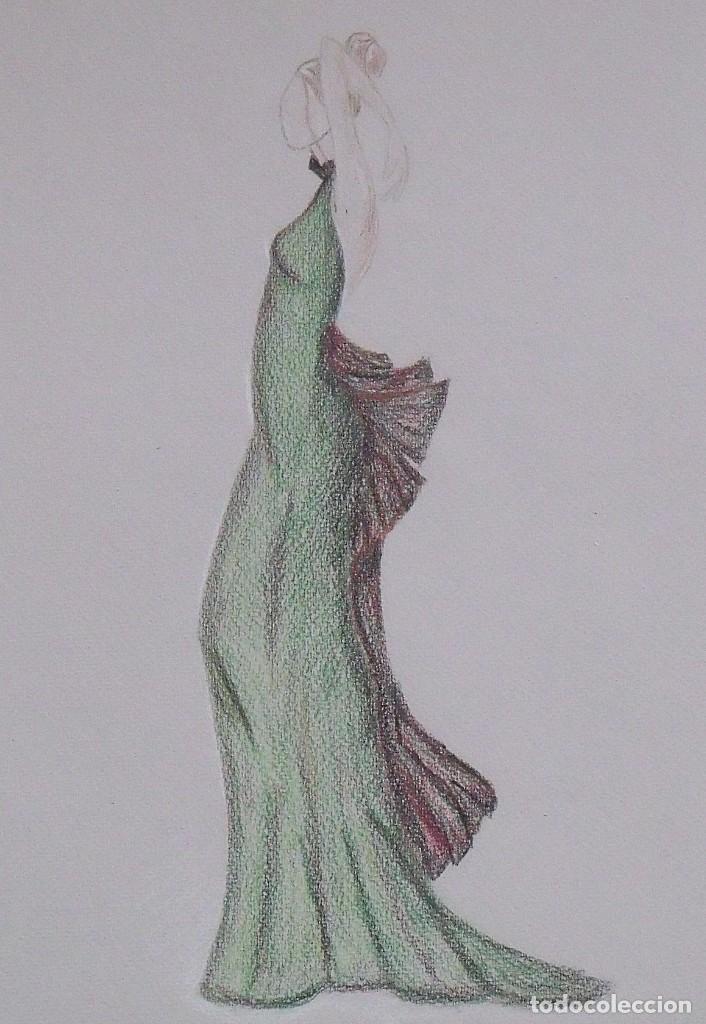
diseño o ilustración de moda. realizada a lápiz - Comprar Dibujos Contemporáneos siglo XX en todocoleccion - 193117875

Mujer mano con lápiz negro vestido de dibujo. Algunos lápices ubicada cerca Fotografía de stock - Alamy

Boceto Dibujado A Mano De La Moda. Mujeres En Trajes De Noche De Colores. Dibujo Acuarela Y Lápiz Fotos, Retratos, Imágenes Y Fotografía De Archivo Libres De Derecho. Image 37602698.

60 ideas de Vestidos dibujados a lápiz | ilustración de moda, ilustraciones de moda, figurines de moda

Bosquejo De La Moda. Mujer En Vestido De Color Largo. Dibujo Acuarela Y Lápiz Fotos, Retratos, Imágenes Y Fotografía De Archivo Libres De Derecho. Image 38070685.

Dibujos De Lápiz De Los Vestidos Y De Los Accesorios Stock de ilustración - Ilustración de sombrero, bolso: 38375376

Gráfico De La Mujer En El Vestido Ornamental, Dibujo A Lápiz Sobre Papel, Sepia Y Efecto De La Vendimia Fotos, Retratos, Imágenes Y Fotografía De Archivo Libres De Derecho. Image 57108352.

Una chica con hermoso vestido dibujo a lápiz paso a paso/ Dibujos fáciles y sencillos para principia - YouTube

60 ideas de Vestidos dibujados a lápiz | ilustración de moda, ilustraciones de moda, figurines de moda











