
Amazon.com: Trame: A Contemporary Italian Reader: 9780300124958: Abbona-Sneider, Cristina, Borra, Antonello, Pausini, Cristina: Books
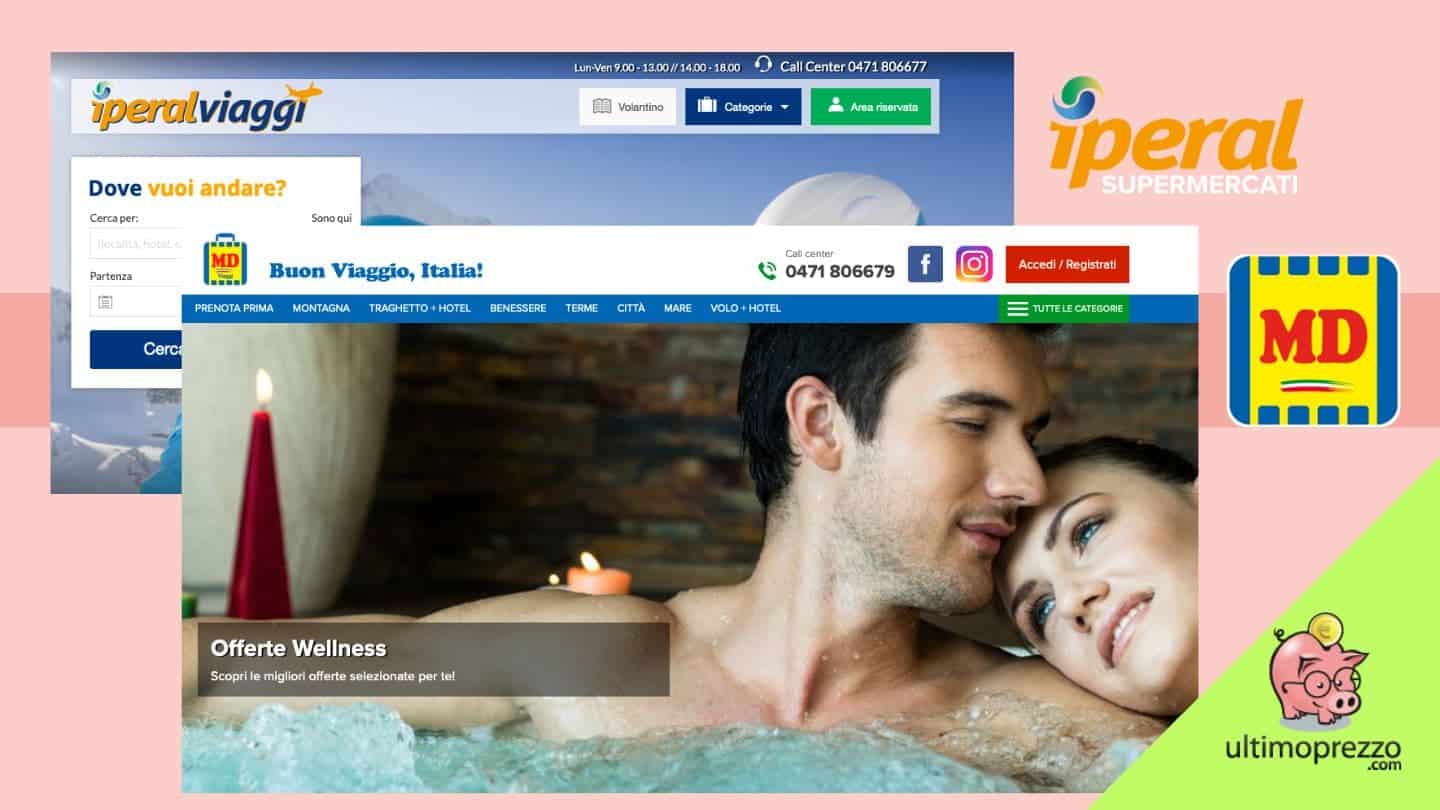
Supermercati per prenotare le vacanze: MD Viaggi e Iperal Viaggi offrono pacchetti per Italia, Europa ed estero, ecco come funzionano - ultimoprezzo.com

Amazon.com: GL.iNet GL-MT300N-V2(Mango) Portable Mini Travel Wireless Pocket VPN Router - WiFi Router/Access Point/Extender/WDS | OpenWrt | 2 x Ethernet Ports | OpenVPN/Wireguard VPN | USB 2.0 Port | 128MB RAM :

Wonderbox - Box Regalo Relax In Due - Il Cofanetto Perfetto Per Regali Di Coppia, Come Idee Regalo Per Anniversario E Come Pacchetto Viaggio Regalo Per Due : Amazon.it: Sport e tempo libero

Selighting Borsa Bicicletta Posteriore Cremagliera 25L Impermeabile di WILDKEN Borsa Bici Multifunzionale Pacchetto Zaino Borsa da Bicicletta per Viaggio : Amazon.it: Sport e tempo libero

Waynabox - Cofanetto Regalo per Viaggio a Sorpresa per 2 Persone con Volo+Hotel per 3 Giorni e 2 Notti in più di 60 Città in Europa : Amazon.it: Sport e tempo libero

TAILI Roll-up Sacchetti Sottovuoto per Vestiti da Viaggio, 10 pacchetti (70×45cm&40×60cm) Sacchi Sottovuoto per coperte, abiti, viaggi o traslochi, senza pompa necessaria Buste Sottovuoto Vestiti : Amazon.it: Casa e cucina

Amazon.com: GL.iNet GL-MT300N-V2(Mango) Portable Mini Travel Wireless Pocket VPN Router - WiFi Router/Access Point/Extender/WDS | OpenWrt | 2 x Ethernet Ports | OpenVPN/Wireguard VPN | USB 2.0 Port | 128MB RAM :

Waynabox - Cofanetto Regalo per Viaggio a Sorpresa per 2 Persone con Volo+Hotel per 3 Giorni e 2 Notti in più di 60 Città in Europa : Amazon.it: Sport e tempo libero

GL.iNet GL-MT300N-V2(Mango) Portable Mini Travel Wireless Pocket VPN Router - WiFi Router/Access Point/Extender/WDS | OpenWrt | 2 x Ethernet Ports | OpenVPN/Wireguard VPN | USB 2.0 Port | 128MB RAM : Electronics - Amazon.com

Waynabox - Cofanetto Regalo per Viaggio a Sorpresa per 2 Persone con Volo+Hotel per 3 Giorni e 2 Notti in più di 60 Città in Europa : Amazon.it: Sport e tempo libero

Amazon.com: GL.iNet GL-MT300N-V2(Mango) Portable Mini Travel Wireless Pocket VPN Router - WiFi Router/Access Point/Extender/WDS | OpenWrt | 2 x Ethernet Ports | OpenVPN/Wireguard VPN | USB 2.0 Port | 128MB RAM :

Amazon.com: GL.iNet GL-MT300N-V2(Mango) Portable Mini Travel Wireless Pocket VPN Router - WiFi Router/Access Point/Extender/WDS | OpenWrt | 2 x Ethernet Ports | OpenVPN/Wireguard VPN | USB 2.0 Port | 128MB RAM :

Waynabox - Cofanetto Regalo per Viaggio a Sorpresa per 2 Persone con Volo+Hotel per 3 Giorni e 2 Notti in più di 60 Città in Europa : Amazon.it: Sport e tempo libero










